Evaporation vs Boiling: Key Distinctions for Science Students
- 13 September 2025

In Primary 5 Science, students are introduced to how water changes between states in the Cycles in Matter and Water (Water) chapter. They learn to describe how water can gain or lose heat to become a gas, liquid, or solid, setting the foundation for two often-confused processes: evaporation and boiling.
Both involve a liquid turning into gas, but that’s where the similarity ends. The way they happen, the conditions required, and the effects produced are quite different.
Students must not only recognise these distinctions but carry them forward as they progress into Secondary Science. This is because concepts like boiling point and rate of evaporation continue to reappear in chapters such as Exploring Diversity of Matter by its Physical Properties and Separation Techniques, where a clear understanding of phase changes supports more advanced problem-solving.
So how exactly do these processes differ and why do those differences matter so much in your child’s learning journey?
What is Boiling?
Boiling is a rapid change of state where a liquid turns into gas once it reaches a specific temperature, which is known as its boiling point. For pure water, this happens at 100°C under normal atmospheric conditions. Unlike evaporation, which happens quietly and only at the surface, boiling is energetic and takes place throughout the entire liquid.
At this point, the liquid molecules have gained enough kinetic energy to break free from the forces holding them together in the liquid state. This is when you see the familiar bubbling action, evidence that the liquid is changing into vapour and escaping into the air.
How Boiling Occurs
Boiling is not just a mere surface-level bubbling. The change of states from liquid to water vapour happens when:
- Heat is applied to the liquid, increasing the energy of the molecules.
- Temperature rises until it hits the boiling point.
- Vapour pressure builds to match the surrounding atmospheric pressure.
- Bubbles form throughout the liquid as molecules break free into gas.
- Bubbles rise and burst at the surface, releasing steam.
This process continues steadily as long as the heat source remains.
What is Evaporation?
Evaporation is the gradual change of a liquid into gas that happens quietly at the surface of the liquid. Unlike boiling, it doesn’t require the liquid to reach a specific temperature, as it can take place even on a cool day. As long as heat is present, some high-energy molecules at the surface of the liquid will escape into the air as vapour.
This process may not always be visible, but it’s happening around us all the time, from sweat drying off your skin to puddles disappearing after rain.
How Evaporation Occurs
Evaporation is a slow but constant process that takes place without needing to hit the boiling point:
- Molecules at the surface of the liquid absorb heat from their surroundings.
- Energy builds up, causing some molecules to move fast enough to break away.
- These surface molecules escape into the air as vapour.
- The process happens at any temperature, not just when the liquid is hot.
Because only surface molecules are involved, evaporation is slower than boiling.
Comparing the Two Processes: What is the Difference Between Evaporation and Boiling
So, how is evaporation different from boiling if both turn a liquid into gas?
While the end result might look similar, the way each process unfolds is quite different. From the temperature at which they happen to where in the liquid they occur, there are a few differences that show how heat affects liquids in distinct ways.
1. The Temperature of Occurrence
Though both involve heat, boiling and evaporation happen under very different temperature conditions:
- Boiling happens at a fixed temperature, known as the boiling point. For pure water, this is exactly 100°C.
- Evaporation can occur at any temperature, even on cooler days. The liquid doesn’t need to reach its boiling point for surface molecules to escape as gas.
The key takeaway: Boiling is quick and temperature-specific, while evaporation is slower and always in progress, provided there’s enough heat.
2. Where the Change Occurs
While both processes result in a liquid turning into gas, the transformation doesn’t happen in the same place within the liquid.
- Boiling affects the entire liquid. Bubbles form at the bottom and rise as heat is evenly distributed.
- Evaporation takes place only at the surface, where molecules have direct contact with the air.
The key takeaway: Boiling happens throughout the liquid, while evaporation is limited to the surface.
3. The Speed of the Process
The pace at which the liquid turns into gas also helps set boiling and evaporation apart.
- Boiling is a rapid process. Once the liquid hits its boiling point, the change happens quickly throughout.
- Evaporation is much slower, unfolding gradually over time as surface molecules gain energy.
The key takeaway: Boiling is fast and dramatic; evaporation is slow and subtle.
4. The Source of Heat
How heat is absorbed differs greatly between boiling and evaporation.
- Boiling needs a direct heat source, like a stove or kettle, to push the liquid to its boiling point.
- Evaporation relies on ambient heat from the surroundings, such as sunlight or warm air.
The key takeaway: Boiling demands strong, focused heat, evaporation makes use of whatever warmth is available in the environment.
Factors that Influence Evaporation
If evaporation can happen at any time, does that mean it’s always happening, even during boiling? And why does a puddle dry up faster at noon than in the evening? These kinds of questions come up often when students try to grasp how evaporation behaves differently from boiling. While the process may seem passive, several factors can affect how quickly or slowly it happens.
1. Temperature
The higher the temperature, the faster evaporation tends to occur. This is because heat gives liquid particles more energy, making it easier for them to escape into the air.
The key takeaway: Warmer surroundings give water molecules the energy boost they need to evaporate faster.
2. Surface Area
Beyond temperature, how the liquid is spread out can also make a big difference. The more surface that’s exposed to air, the quicker evaporation takes place. This means that a wider surface gives more molecules direct access to the air, allowing them to escape faster.
The key takeaway: The greater the exposed surface, the more opportunities there are for evaporation to happen.
3. Wind Speed
Still air slows evaporation down, but movement in the air can tip the balance. A breeze, for instance, can help carry water molecules away as the wind removes the evaporated water vapour lingering above the surface, making space for more molecules to escape. This creates a constant cycle that speeds up the process, especially on windy or breezy days.
The key takeaway: Wind helps clear away water vapour, keeping the evaporation process going strong.
The Role of Kinetic Energy
So, how exactly do molecules manage to escape the liquid state in both boiling and evaporation? It all comes down to kinetic energy. As heat is absorbed, molecules start moving faster. When they gain enough energy, they can break free from the bonds holding them in the liquid.
- During boiling, the entire liquid reaches this energy threshold at once, leading to rapid bubbling throughout the liquid.
- During evaporation, only surface-level molecules gradually gain enough energy to escape, which is why the process happens much more slowly and without bubbling.
Practice Questions on Evaporation and Boiling

Ready to put your understanding of the differences between evaporation and boiling to the test?
1. Multiple-Choice Question
Question: Which of the following statements about evaporation is incorrect?
- Evaporation can happen even when water is not boiling.
- Evaporation takes place throughout the liquid.
- Evaporation is a slower process than boiling.
- Evaporation occurs more quickly when it’s warm and windy.
Answer: (2) is incorrect.
Evaporation occurs only at the surface of a liquid, unlike boiling, which happens throughout. The other statements correctly describe how evaporation works in everyday situations.
2. Experimental Analysis Question
Question: Jamie set up two identical beakers filled with the same amount of water. In one setup, the beaker was placed on a hot plate. In the other, it was left at room temperature. After recording the temperature and volume of water at regular intervals, she plotted two graphs.
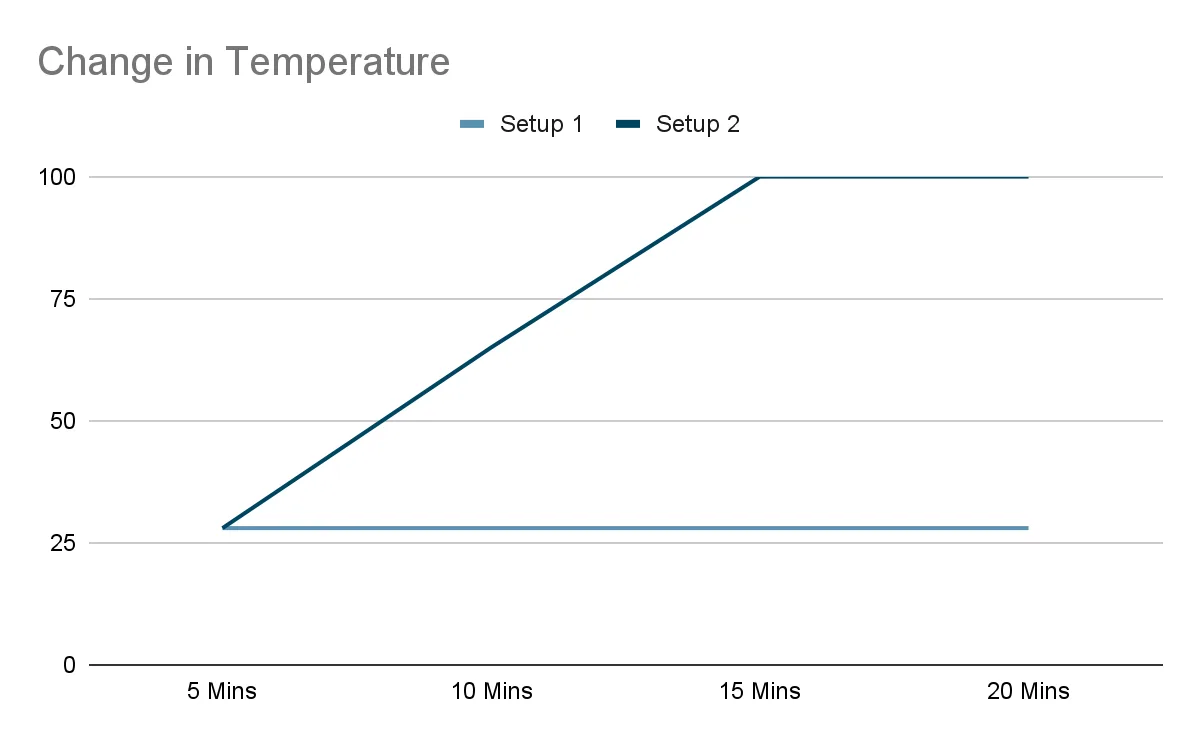
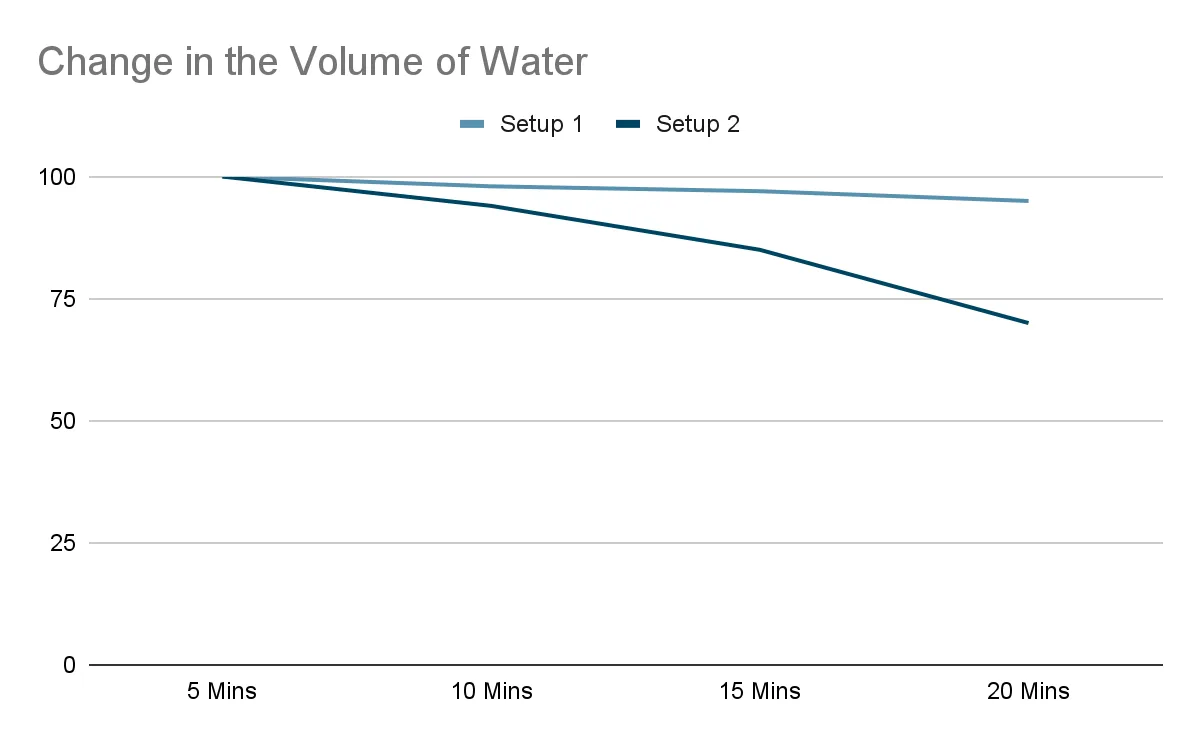
Which setup had the hot plate switched on? Give two pieces of evidence from the graphs to support your answer.
Answer:
The hot plate was switched on in setup 2, where:
- The temperature graph shows a steady increase before holding constant at 100°C, indicating boiling.
- The volume of water decreases more rapidly over time, suggesting a faster change of state due to continuous heating.
These patterns point to boiling taking place, as opposed to gradual evaporation in the setup left at room temperature.
Mastering concepts like evaporation and boiling builds a solid foundation for tackling more complex topics ahead. At TLS Tutorials, our Science tuition for Primary 5 and Primary 6 students goes beyond memorisation. We equip students with the tools they need to make sense of real-world phenomena. If you’re looking for Primary School Science tuition in Singapore that bridges the gap between understanding and application, TLS Tutorials is ready to guide your child every step of the way.
